
Phase III postneoadjuvant study evaluating Sacituzumab Govitecan, an Antibody Drug Conjugate in primary HER2-negative breast cancer patients with high relapse risk after standard neoadjuvant treatment - SASCIA
Phase III postneoadjuvant study evaluating Sacituzumab Govitecan, an Antibody Drug Conjugate in primary HER2-negative breast cancer patients with high relapse risk after standard neoadjuvant treatment - SASCIA
A Study of the German Breast Group (GBG) in collaboration with the AGO-B
EudraCT-No.: 2019-004100-35
Introduction
This is a Phase III, prospective, multi-center, randomized, open label, parallel group, study evaluating the potential incremental efficacy and safety of Sacituzumab Govitecan in high-risk patients with HER2-negative breast cancer with residual disease (TNBC or HR-positive) after neoadjuvant chemotherapy. A total of 1322 patients will be included in the study.
News
The SASCIA study has been transferred to the Clinical Trials Regulation (536/2014, CTR). EU CT number: 2023-510390-33-00
The treatment phase was completed on 09.09.2024. The study is now in follow-up.
Design
All Patients must have received neoadjuvant taxane-based chemotherapy for 16 weeks (anthracyclines are permitted). This period must include 6 weeks of a taxane containing neoadjuvant chemotherapy.
After NACT adequate surgical treatment including resection of clinically evident disease and ipsilateral axillary lymph node dissection should be performed. Patients should have residual invasive disease at surgery after NACT.
The patients will be randomized with 1:1 allocation to:
Arm A: Sacituzumab Govitecan 10 mg / kg per IV (days 1, 8 q3w for eight cycles) IMP Arm
OR
Arm B: Treatment of physician´s choice:
- Capecitabine 2000 mg/m² day 1-14 q21 day cycle for eight cycles or
- Carboplatin AUC 5 q3w or AUC 1.5 weekly for eight 3 weekly cycles or
- Observation.
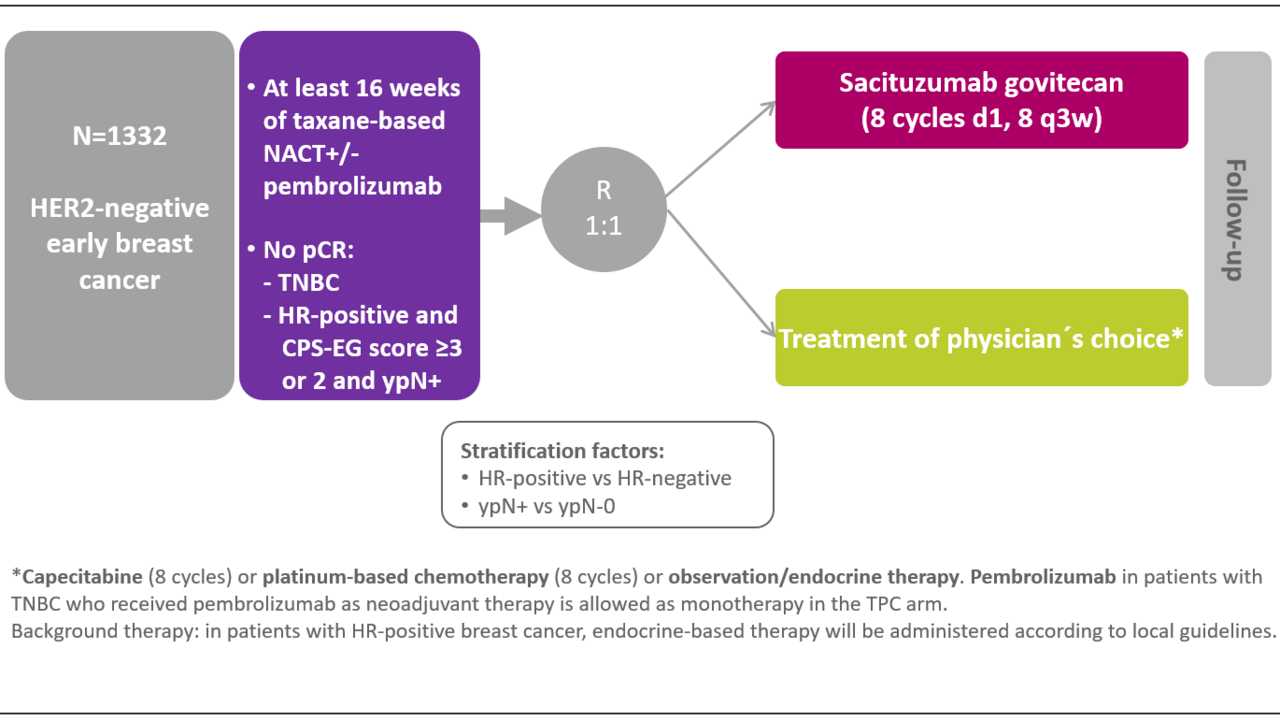
The inclusion and exclusion criteria, as well as more detailed information on the study design, can be found in the synopsis on the list of documents.
Contact
Dr. Laura Steinmann
