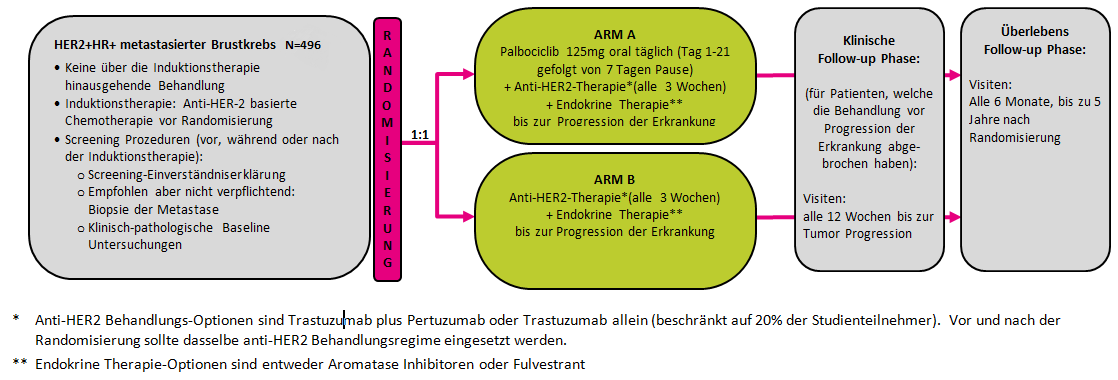
A Randomized, Open Label, Phase III Trial to Evaluate the Efficacy and Safety of Palbociclib + Anti-HER2 Therapy + Endocrine Therapy vs. Anti-HER2 Therapy + Endocrine Therapy after Induction Treatment for Hormone Receptor Positive (HR+)/HER2-Positive Metastatic Breast Cancer
EudraCT#: 2017-000419-17
ClinicalTrials.gov Identifier: NCT02947685
Protocol Number: AFT-38
US Sponsor Name: Alliance Foundation Trials (AFT), LLC
News
Status: Recruitment completed
Last Patient Last Visit expected: Apr 2026
Design
Contact
Project management
patina@gbg.de
+496102 / 7480-440