
Evaluation of palbociclib plus endocrine treatment versus a chemotherapy-based treatment strategy in patients with hormone receptor positive / HER2 negative breast cancer in a real world setting.
Evaluation of palbociclib plus endocrine treatment versus a chemotherapy-based treatment strategy in patients with hormone receptor positive / HER2 negative breast cancer in a real world setting.
EudraCT-Nr.: 2016-004482-89
Introduction
Endocrine therapy is the recommended option for ER positive / HER2 negative MBC patients as first-line therapy in the majority of patients except those with rapidly progressing, life-threatening disease, also known as visceral crisis. With the novel CDK4/6 inhibitors in addition to either an AI or fulvestrant the treatment landscape is changing rapidly. However, the data comparing endocrine therapy alone with chemotherapy are scarce and less convincing. Since palbociclib improves the efficacy of endocrine therapy alone by about 50%, the hypothesis is that palbociclib + endocrine therapy is superior to mono-chemotherapy of physician´s choice with or without ET maintenance therapy in time to treatment failure.
However, due to rigid inclusion and exclusion criteria, limited number of treatment options, and strictly prescribed monitoring intervals the majority of clinical trials are done in an “artificial environment” and often do not mirror real world situation. Therefore, this trial is planned as so called low intervention real world trial.
The goal of the study is to show that palbociclib + ET shows a significant improvement in time-to-treatment failure (TTF) over chemotherapy regimen (mono-chemotherapy with or without ET maintenance therapy). In addition, we assume that Patient Reported Outcome will be improved with palbociclib + ET vs. CT regimen.
News
Trial is completed.
You can find the summary for patients under Downloads.
Design
A randomized, open-label, multi-center phase IV study evaluating palbociclib plus endocrine treatment versus a chemotherapy-based treatment strategy in patients with hormone receptor positive / HER2 negative breast cancer in a real world setting.
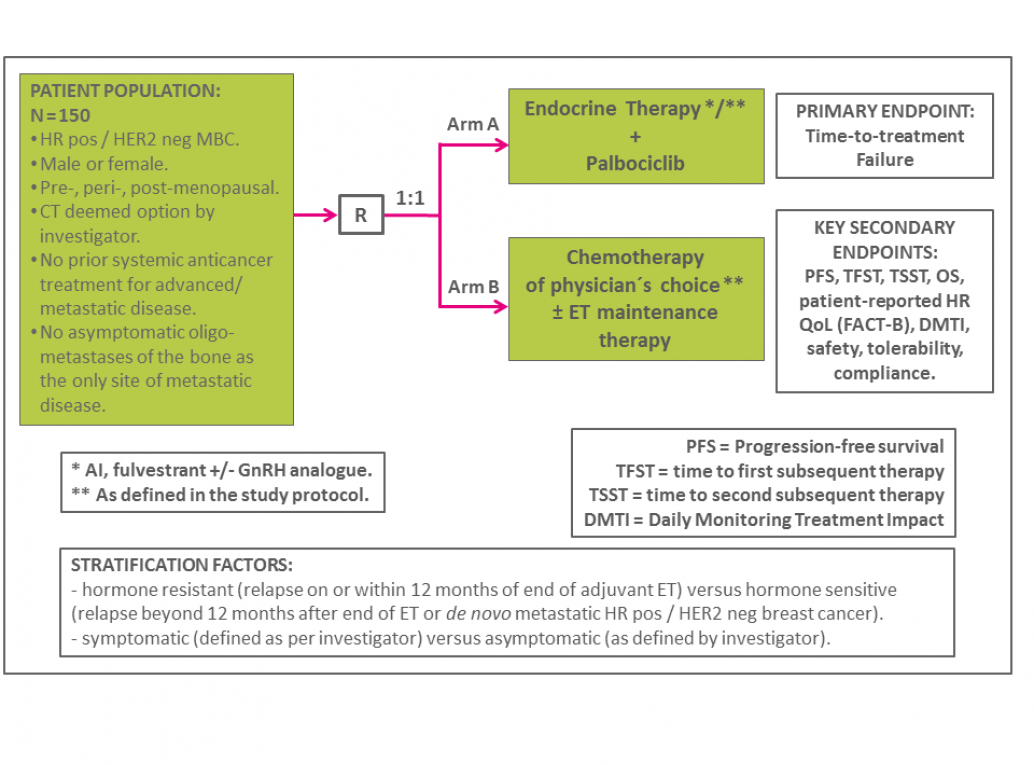
The inclusion and exclusion criteria, as well as more detailed information on the study design, can be found in the short protocol on the list of documents.
Contact
Dr. Thomas Ballhausen
