A randomized, open-label, phase II trial comparing neoadjuvant endocrine therapy in combination with trastuzumab, pertuzumab +/- the PI3K inhibitor inavolisib in patients with HER2-positive, HR-positive, PIK3CA mutant early breast cancer- GeparPiPPa
A randomized, open-label, phase II trial comparing neoadjuvant endocrine therapy in combination with trastuzumab, pertuzumab +/- the PI3K inhibitor inavolisib in patients with HER2-positive, HR-positive, PIK3CA mutant early breast cancer- GeparPiPPa
EudraCT-No.: 2021-002323-38
EU-CT-Number: 2022-501152-28
Introduction
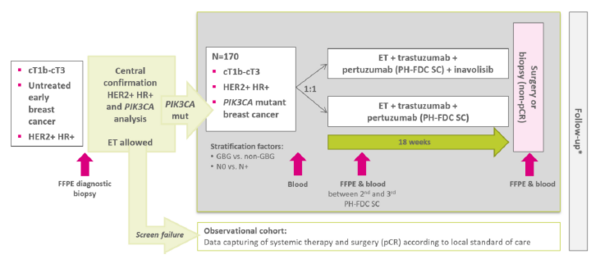
The study evaluates the potential incremental efficacy and safety of inavolisib in the neoadjuvant treatment of early-stage HER2-positive, HR-positive, PIK3CA mutant breast cancer.
170 patients with confirmed eligibility criteria and PIK3CA mutant breast cancer receive a neoadjuvant endocrine therapy in combination with dual anti-HER2 blockade consisting of ready-to-use fixed-dose combination of pertuzumab and trastuzumab as subcutaneous (PH-FDC SC) formulation q3w for 6 cycles (18 weeks), randomized in a 1:1 ratio with or without Inavolisib.
Endocrine therapy consists of either tamoxifen 20mg or an aromatase inhibitor +/- GnRH analogue for premenopausal women and men.
All patients will undergo surgery or biopsy after completing study therapy to assess pCR rate. In case of ycT0 and no tumor residuals in the biopsy, it is recommended to undergo surgery; in case of tumor residuals in the biopsy further neoadjuvant treatment may be given.
News
Patient recruitment has started. The first patient was registered in January 2023.
You are welcome to refer a patient to one of our trial sites. Interested physicians can view an overview of all participating sites on the new Reesi platform.
Here you will find the link to the login area.
The study has also started recruiting in Italy since November 2024. The first patient from Italy was randomized on 17-Jan-2025.
Since April 2025, the study has also been started recruiting in Spain. The first patient from Spain was randomized on May 6, 2025.

The inclusion and exclusion criteria, as well as more detailed information on the study design, can be found in the short protocol on the list of documents.
Contact
Jana Roßney