
Neo- / adjuvant phase III trial to compare intense dose-dense chemotherapy to tailored dose-dense chemotherapy in patients with high risk early breast cancer.
Introduction
EudraCT No.: 2011-005214-11
Status: Study is in Follow-up
A Neo- / adjuvant phase III trial to compare intense dose-dense chemotherapy to tailored dose-dense chemotherapy in patients with high risk early breast cancer.
News
Since 01.08.2016 the amendment 3 is effective. Currently 1994 patients are randomized.
GAIN-2 Trastuzumab s.c. Substudy
HER2-positive patients can participate in the trastuzumab subcutaneous sub-study after completion of the GAIN-2 main study, in which the antibody is injected either into the abdominal wall or into the thigh.
Please note that randomization into the sub-study can only be performed after complete documentation of the main study. In addition, the last trastuzumab infusion during the GAIN-2 main study must be 6 mg kg/KG q3w. For the subcutaneous injection during the sub-study, 600 mg trastuzumab q3w is then always administered.
Trastuzumab will be administered for a total of one year.
Please ask your HER2-positive GAIN-2 patients about the trastuzumab s.c. sub-study - a total of 220 patients are to be included in this sub-study. All GAIN-2 centers can participate.
Design
A Neo- / adjuvant phase III trial to compare intense dose-dense chemotherapy to tailored dose-dense chemotherapy in patients with high risk early breast cancer.
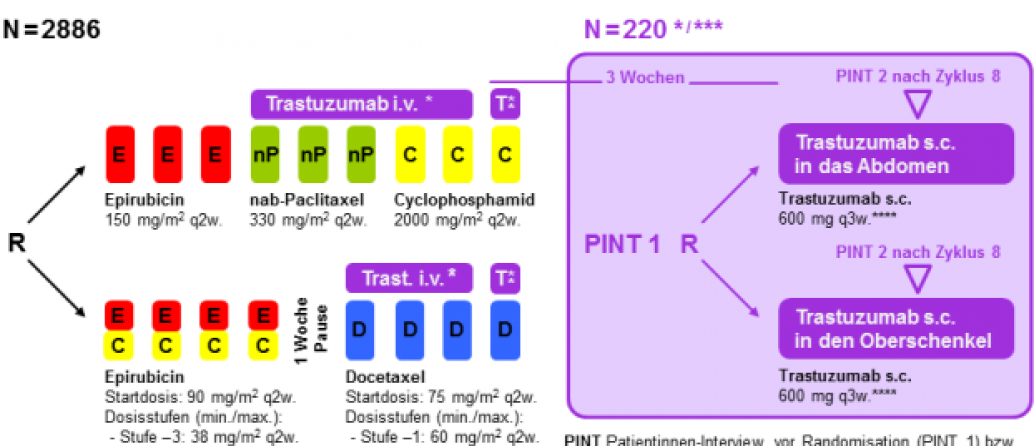
The inclusion and exclusion criteria as well as more detailed information on the study design can be found in the short protocol on the documents page.
Contact
Konstantin Reißmüller
