
Introduction
A Study of Camizestrant in ER+/HER2- Early Breast Cancer After at Least 2 Years of Standard Adjuvant Endocrine-Based Therapy.
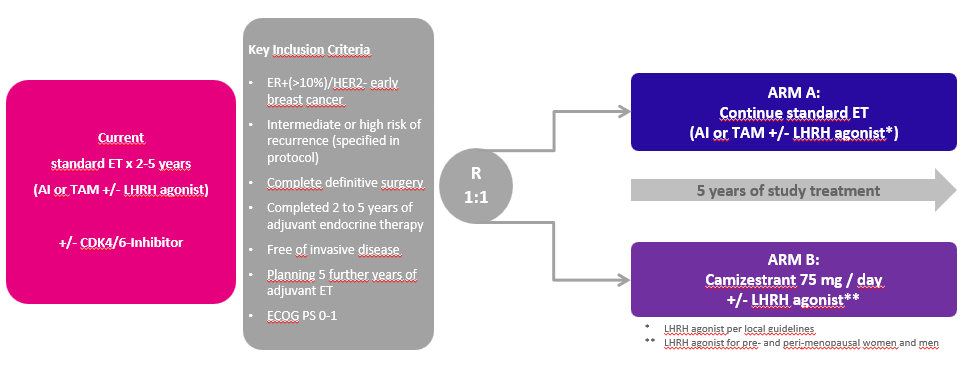
The target population of interest in this study consists of male and female patients with ER+/HER2- early breast cancer, who have completed definitive locoregional therapy and at least 2 years of standard adjuvant ET, with or without a cyclin dependent kinase 4 and 6 (CDK4/6) inhibitor, and who are currently disease-free but with intermediate or high risk of recurrence.
News
We are still looking for patients for this study. You are welcome to refer a patient to one of our trial sites. Interested physicians can view an overview of all participating sites on the new Reesi platform.
Here you will find the link to the login area.
Details about the study, inclusion and exclusion criteria and participating trial sites can be found on ClinicalTrials.gov.
Patients can find a flyer with information for patients in the download area on the right. We gladly inform you of the nearest study site on request.

Design
Primary Efficacy
Comparison of IBCFS (invasive breast cancer-free survival) rates by treatment
Patient Profile
- Early ER+/HER2- breast cancer with no disease recurrence
- Pre-, Peri-, and postmenopausal Women and Men
- Adequate surgical and systemic pre-therapy with or without chemotherapy or radiotherapy
- At least 2 years and up to 5 years of standard adjuvant ET
- Patients may have received adjuvant CDK4/6 inhibitors prior to study entry
- Intermediate or high risk of recurrence as defined per protocol
More downloads
Contact
Anna Huber
