Since foundation of GBG, we have worked with 800 study centres around the world and centres around the world and have treated more than 67,000 patients in > 115 studies.
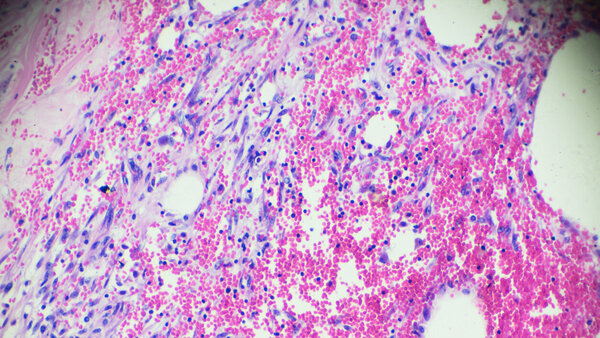
We are researching breast cancer treatment in academic phase II to IV clinical trials.
phase II to IV clinical trials. The hypotheses for potential trials are potential studies are developed in regular meetings with the sub-board members. This means that at the end of an exchange between our independent, external clinical experts an idea is developed which is then investigated in studies. These These study concepts are planned, made publicly available and quality standards, made publicly accessible and carried out in order to transparency about compliance with the standards at all times.To this end, we also cooperate closely with research-based pharmaceutical manufacturers and combine state-of-the-art therapy options in order to find the best treatment recommendations.
Our research area includes the following forms of therapy or types of treatment:
- Pre-operative (neoadjuvant) drug therapy
- local therapy (surgery & radiotherapy)
- post-operative (adjuvant) therapy
- Drug therapy for chronic breast cancer
- Prevention